The reports notes that “… more than 80 percent of active pharmaceutical ingredients—the building blocks of medicines—are imported, and 40 percent of medicines are imported as finished products. Further, U.S. imports of medical devices quadrupled over the last 10 years…”

Making the case for personal importation of safe, affordable prescription medicines from licensed, registered pharmacies in Tier One Countries. Rx for American Health is published by Daniel Hines, an international award-winning communicator with five decades of experience, and the publisher of www.TodaysSeniorsNetwork.com and www.BoomersNewsOnline.com. He also works with progressive senior advocacy groups across the nation to promote the health and well-being of America’s aging population.
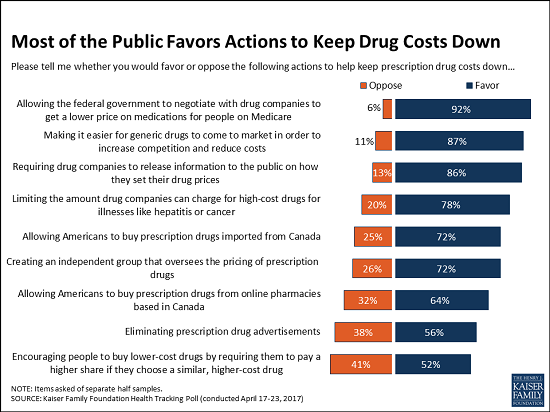
Kaiser Poll Show Support for Personal Imporatation
Monday, April 16, 2012
Recent findings of two separate studies should nudge FDA to reciprocity
The reports notes that “… more than 80 percent of active pharmaceutical ingredients—the building blocks of medicines—are imported, and 40 percent of medicines are imported as finished products. Further, U.S. imports of medical devices quadrupled over the last 10 years…”
Monday, April 2, 2012
Independent Study confirms safety and cost-effectiveness of personal importation
St. Louis, MO, April 2, 2012--The publisher of a leading informational website for America’s seniors, caregivers and policy-makers says that an independent study by the National Bureau of Economic Research validates the claims of supporters and advocates that personal importation of brand-name prescription medicines from reputable sources outside the U.S. is both safe and cost effective.
Daniel Hines, publisher of www.TodaysSeniorsNetwork.com, says the study, ‘Unveiling the Mystery of Online Pharmacies: an Audit Study ‘ should serve as a guide for policy-makers, including elected officials and the Food and Drug Administration (FDA), to ensure that facts about the safety, efficacy and cost-savings of personal importation of prescription medicines are not buried under the efforts by the Pharmaceutical industry (Pharma) attempts to ‘lump’ legitimate sources of safe, affordable medicines from outside the U.S. with bogus, illegitimate sources.
Legitimate sources of prescription medicines for personal importation of brand-name medicnes into the U.S. have established their own rigorous standards of safety and efficacy, Hines says.
“U.S. citizens pay the highest cost in the world for their prescription medicines, a reflection of the success of Pharma in influencing policy that has made the country a ‘safe haven’ for medicines,” Hines says.
In the study, the investigators purchased four top-selling brand name medicines from U.S. pharmacies and via personal importation. The medicines from the U.S. pharmacies cost an average of 52.4 percent more.
“The newly released study illustrates that the attempts by Pharma to impugn the safety, efficacy and savings of brand-name prescription medicines from such legitimate sources outside the U.S. by a more than decade-long effort to cast them in the same light as bogus operations that have no regard for safety or health, and that provide counterfeit medicines without prescriptions has been misdirected,” Hines says.